Abstract
Background: Allogeneic HCT is the standard of care for eligible patients with AML harboring high-risk features such as adverse-risk cytogenetics or minimal residual disease (MRD). However, the majority of such patients still relapse post-HCT and have very poor outcomes. The use of maintenance therapies intended to control post-HCT relapse is often limited by hematopoietic toxicity towards normal engrafted cells. Gemtuzumab ozogamicin (GO, tradename: Mylotarg) is an FDA-approved anti-CD33 antibody-drug conjugate targeting CD33+ AML, but its use is limited by on-target hematopoietic toxicity towards CD33+ myeloid and progenitor cells. VOR33 is a CRISPR/Cas9 gene-edited allogeneic hematopoietic stem and progenitor cell (HSPC) product lacking CD33 protein that was developed to protect normal hematopoietic cells while exclusively targeting leukemia with CD33-directed therapies. VOR33 is manufactured from CD34+ cells isolated from healthy human leukocyte antigen (HLA)-matched donors.
Pre-clinical murine and primate transplant studies using CD33-deleted HSPCs demonstrated unaltered long-term engraftment, multi-lineage hematopoietic differentiation and function, as well as long-term persistence of CD33 gene-edited hematopoietic cells. Additionally, subsequent treatment with GO eradicated CD33+ leukemia cells while the normal hematopoietic cells that reconstituted from CD33-edited HSCs were preserved. The improved therapeutic index for GO in combination with VOR33 has the potential to allow effective targeting of residual leukemia in AML patients post-HCT without dose-limiting hematopoietic toxicity.
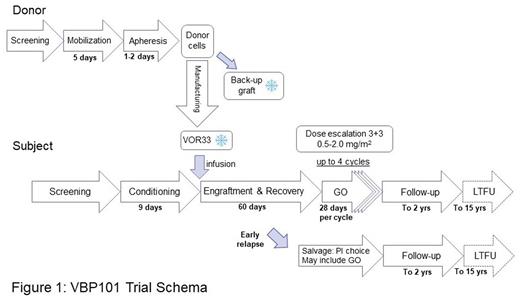
Study Design and Methods: VBP101(NCT04849910) is a first-in-human Phase 1/2 open-label multicenter trial to establish the safety of using VOR33 as a donor allograft for CD33+ AML patients who are at high risk of relapse and undergoing myeloablative HCT followed by treatment with low-dose GO.
Participants (18-70 y) must have a diagnosis of CD33+ AML, an HLA-matched related or unrelated (10/10) donor and be a candidate for myeloablative HCT. Additional key inclusion criteria include CR1/CRi/CR2 with intermediate/poor cytogenetics and the presence of MRD. Patients with 5-10% bone marrow-only blasts with any cytogenetic risk are also eligible. Key exclusion criteria include prior auto/allo-HCT or prior treatment with GO.
Related and unrelated donors undergo mobilization with G-CSF and plerixafor prior to cell collection. Manufacturing of VOR33 and isolation of an unmanipulated backup graft are performed centrally and both are cryopreserved. Subjects undergo either a busulfan- or TBI-based myeloablative conditioning regimen prior to transplantation with VOR33. After engraftment and recovery of a sufficient CD33-negative neutrophil count (~Day 60), patients begin maintenance therapy with GO.
Part 1 of the study will enroll 9-18 evaluable patients to be treated in a 3+3 dose-escalation strategy with GO (0.5-2.0 mg/m2) in a 28-day cycle for up to 4 cycles. Part 1 will evaluate the safety of VOR33/GO and will determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of GO. The Part 2 expansion phase will enroll an additional 12 patients to further evaluate the safety of VOR33 and the preliminary efficacy of VOR33 and GO at the RP2D. A separate cohort is included for early relapse with treatment per the investigator's choice and may include use of GO. The primary endpoint is to assess the safety of VOR33 as measured by successful neutrophil engraftment at 28 days post-HCT. Secondary endpoints include time to neutrophil and platelet engraftment, assessment of the overall safety of VOR33 and GO (GvHD, graft failure, transplant-related mortality, RP2D, and MTD), non-hematological GO-related toxicities, GO pharmacokinetics, the efficacy of VOR33 in conferring protection from GO-related hematological toxicities, percent CD33 negative myeloid cells over time and survival rates (RFS and OS). Exploratory endpoints include the persistence of VOR33 gene editing and immune reconstitution. Study follow-up is 2 y with up to 15 y long term follow-up in a rollover safety study, VBP201 (NCT05309733). A Dose Escalation Committee and Data Safety Monitoring Committee will review GO dosing levels and monitor overall trial safety, respectively. VBP101 is currently open for enrollment and recruiting patients.
Disclosures
Koura:Cidara: Consultancy. Bambace:AbbVie: Consultancy; Bristol Myers Squibb: Consultancy. Walter:Boston Biomedical, Inc: Consultancy; Astellas Pharma US, Inc: Consultancy; Selvita: Research Funding; Celgene, Inc: Consultancy, Research Funding; Kronos Bio, Inc: Consultancy; BerGenBio, ASA: Consultancy; Bristol Myers Squibb, Inc: Consultancy; BioLineRx, LTd: Consultancy, Research Funding; Genentech: Consultancy; GSK: Consultancy; ImmunoGen: Research Funding; Janssen Global Services, LLC: Consultancy; Janssen Research and Development: Research Funding; Pfizer, Inc: Consultancy, Research Funding; Race Oncology LTD: Consultancy; Orum Therapeutics, Inc.: Consultancy; Aptevo Therapeutics: Consultancy, Research Funding; Kite Pharma, Inc: Consultancy; Agios: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Amphivena Therapeutics, Inc: Current equity holder in publicly-traded company; New Link Genetics: Consultancy; Arog Pharmaceuticals: Research Funding; AbbVie: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding; Stemline Therapeutics: Research Funding; Kura Oncology: Consultancy, Research Funding; MacroGenics: Consultancy, Research Funding. DiPersio:VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; BioLineRx, Ltd.: Research Funding; Macrogenics: Research Funding; NeoImmune Tech: Research Funding; Amphivena Therapeutics: Research Funding; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; WUGEN: Current equity holder in private company, Research Funding. Nath:Vor Biopharma: Current Employment, Current holder of stock options in a privately-held company. Lee-Sundlov:Vor Biopharma: Current Employment, Current holder of stock options in a privately-held company; Pfizer: Consultancy. Lacy:Vor Biopharma: Current Employment, Current holder of stock options in a privately-held company. Stanizzi:Vor Biopharma: Current Employment, Current holder of stock options in a privately-held company. Kuhn:Vor Biopharma: Current Employment, Current holder of stock options in a privately-held company. Whangbo:Vor Biopharma: Current Employment, Current holder of stock options in a privately-held company. Slapak:Vor Biopharma: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Raffel:Vor Biopharma: Current Employment, Current holder of stock options in a privately-held company.
OffLabel Disclosure:
Gemtuzumab ozogamicin (GO, tradename: Mylotarg), an FDA-approved anti-CD33 antibody-drug conjugate targeting CD33+ AML will be used as a post HCT maintenance therapy
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal